‘At TU/e I investigate innovative methods to enable the large-scale energy storage systems of the future’
Interview with Antoni Forner Cuenca, assistant professor at the department of Chemical Engineering and Chemistry.
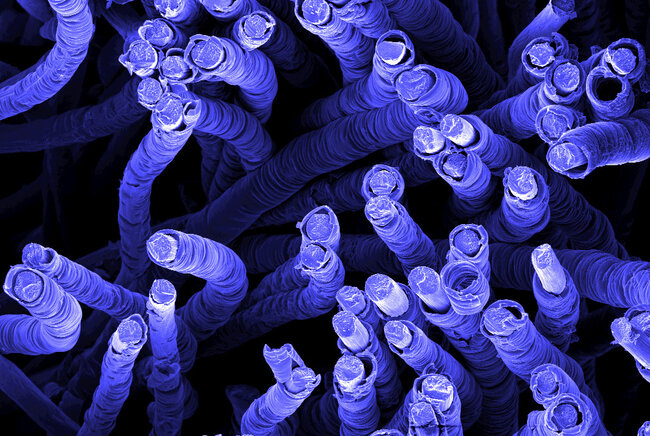
Antoni Forner Cuenca aims for the heart of a battery, more specifically a flow battery which could continuously generate electricity by exploiting chemical reactions between fuels and oxidizing reagents. Forner Cuenca is one of the young talents that were recently awarded a prestigious VENI grant (EUR 250'000) and a grant on innovative future technology (EUR 360'000) by the Netherlands Organization for Scientific Research. In this interview, he shares why flow batteries are promising solutions for large-scale energy-storage and how he hopes to improve their performances.
What attracted you to chemistry originally?
Chemistry is a fascinating discipline. For me it's first and foremost a way of understanding the world; how it's made up and why things work the way they do. What's more, the chemical engineering field allows you to manipulate materials to develop efficient and clean renewable energy technologies.
Like batteries...
The most popular batteries are lithium-ion batteries. These are particularly suitable for all kinds of mobile applications. Yet, their technology is limited in terms of large-scale storage and for prolonged periods of time. For example, they don't scale up well to the larger sizes needed for grid power, or to provide backup power for thousands of houses for many hours. That is why we are working on flow batteries.
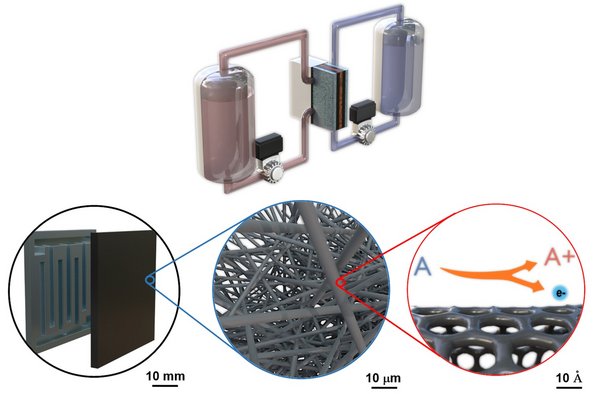
How exactly flow batteries work?
A flow battery consists of two tanks containing a liquid electrolyte. One tank is positively charged, while the other is negatively charged. The liquid is pumped through an electrode and it can be used in charge or discharge mode, reversibly. These flow batteries are promising solutions for large-scale energy-storage because the scaling up simply requires bigger tanks of electrolytes. To date, most flow batteries rely on vanadium as an electrolyte because this element exists in multiple oxidation states and discharges reliably for thousands of cycles. Yet, vanadium is scarce, and its price fluctuates. Therefore, I am focusing on cheaper alternatives that have comparable performances, such as organic compounds, and on improving their efficiency.
And how are you going to do so?
To achieve that, you need to improve the heart of the battery. The magic happens in its electrode, which can collect and release electrons while the liquid electrolyte flows through it. The performance of the battery can be dramatically improved by optimizing the three-dimensional structure of the electrode. If you look at it through a microscope, you’ll see that consists of a porous structure that is poorly organized. We know that working with a very well-structured geometry, instead, leads to greatly improved energy storage systems. Yet, the exact type of structure is not known yet. That’s where I step in with my research.
What will be your approach?
We will use mathematical models to predict the optimal electrode structure. Once we will know the promising electrode morphologies that might lead to the best performance, we will manufacture them by using 3D printing technologies and test the electrodes in the laboratory in close collaboration with industrial partners.
Besides flow batteries, you are currently focusing on other technologies for clean mobility and energy conversion. Can you tell us more?
We investigate polymer electrolyte fuel cells, namely devices that convert the chemical energy of the fuel directly into electrical energy. These devices can be used as power sources for sustainable mobility and offer emission-free and fast-refilling solutions. Also, we focus on electrolyzers, namely devices that use an electric current to split a water molecule into hydrogen and oxygen. These devices have a positive and negative side, like a battery. Hydrogen gas is generated on the negative side while oxygen gas is generated on the positive side. We used electrolyzers to generate hydrogen for powering hydrogen fuel cells and the chemical industry. Enabling a fully renewable energy economy necessitates innovations in mobility, energy storage to integrate renewables, and chemical manufacturing. We use the principles of electrochemical engineering, materials science, and computational modeling to develop a range of electrochemical energy technologies for these three sectors.
What is the overall goal of your research?
We do fundamental, curiosity-driven research. First and foremost, we are striving for a better understanding of what goes on in such a battery and how you can improve the process. We expect to take major steps in this direction within the next three years. At the same time, of course, we’d love to see our research findings its way to the market in the form of valuable new products. Collaborating with the industry and transferring our knowledge about designing and producing electrodes is therefore very important.
What brought you to TU/e?
Before coming to the Netherlands, I had been thinking for a while about the next step in my career. I knew I wanted to become a professor and a leader in my field. And I needed the right place to make that happen. I chose Eindhoven University of Technology because it is a fantastic and renowned university that collaborates intensively with industry within one of the most interesting high-tech regions of Europe. Currently, I am an assistant professor within the Membrane Materials & Processes group, at the Department of Chemical Engineering and Chemistry. At TU/e, I am excited to develop innovative methods to enable the large-energy scale storage systems of the future.
Part of this interview was previously written and published on 'Smart Storage Magazine' by Marco de Jonge Baas.
